Candium Lab
Purpose:
- To use a Candium model to explain the concept of atomic mass.
- To analyze the isotopes of Candium and calculate its atomic mass.
Hypothesis: We hypothesized that the Gobstoppers would have a higher atomic mass and the Sixlets would have the lowest atomic mass because of their sizes.
- 4 Sample of Candium
- Triple Beam Balance
Procedure:
- Obtain 4 samples of Candium
- Separate it into its 4 isotopes (M&M's, Skittles, Sixlets, & Gobstoppers)
- Determine the total mass of each isotope.
- Count the number of each isotope.
- Record data and calculations in the data table.
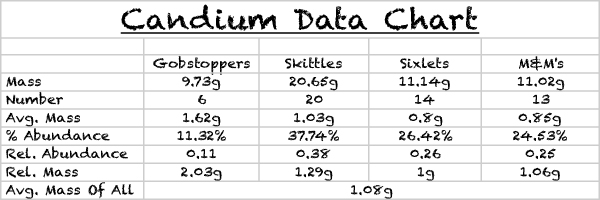
Create a data table that has the following:
1. Average mass of each isotope
2. Percent abundance of each isotope
3. Relative mass of each isotope
4. Relative abundance of each isotope
5. Average mass of all isotopes
Your data table should have five columns and seven rows.
A mix of the samples on the triple beam balance
Isotope- One of two or more atoms having the same atomic number but different mass numbers.
Relative abundance is a comparison between the isotopes and percent abundance is comparing the totals.
The errors that could of happened was we could have done the calculations wrong or we could have counted the samples wrong and all the data would've of been wrong.
Conclusion: We have accepted our hypothesis because the atomic mass of the Gobstoppers was 1.62g, which was much larger than any of the other samples of candy.
Pennium Lab
Purpose: To investigate the concept of atmoic mass and how it was derived. You will develop your own unit of measure CMU (coin mass unit) and use it to measure relative masses of other coins.
Materials:
1. A packet of pennies.
2. 1 quarter, nickel, dime
1. A packet of pennies.
2. 1 quarter, nickel, dime
Procedure:
- Obtain a packet of pennies.
- Sort the pennies into two groups: pre 1982 and post 1982.
- Measure the mass (in grams) of each stack of pennies. Record the masses in a data table
- Count the number of pennies in each stack.
- Measure the mass in grams of a quarter, nickel, and dime. Record these values in the data table.
- Determine the average mass of pre-1982 pennies. 3.05grams
- Determine the average mass of post-1982 pennies. 2.46grams
- Choose one of your coins to make CMU. Let's say that the mass of a nickel is one CMU. Use the mass of a nickel to calculate the mass of a quarter, nickel dime, pre and post 1982 pennies. (make sure you always record your data).
- Determine the average mass.
Questions:
- Does each penny have the same mass? No they all have different masses.
- Can you identify two penny isotopes based on the masses of the pennies? Pre 1982 and post 1982 because their mass values are different so they were most likely made with different elements.
- What does your data tell you about the relationship between mass of a penny and date of a penny? It tells us that the compostition consisted of different materials.
Discussion:
The average mass of the pre and post pennies is 25.97 grams.
My weight in CMUs is 22049995.28 CMU
How I calculated that was took the Nickel which CMU is 1 and it weighed 4.72 so I calculated my weight into kg which I weighed 100lbs. then I converted that to grams then subtracted 4.72 from that number and got my answer.
The average mass of the pre and post pennies is 25.97 grams.
My weight in CMUs is 22049995.28 CMU
How I calculated that was took the Nickel which CMU is 1 and it weighed 4.72 so I calculated my weight into kg which I weighed 100lbs. then I converted that to grams then subtracted 4.72 from that number and got my answer.
Conclusion:
We decided to use the Nickel as the CMU which meant that a CMU weight would be 4.72 grams. The mass of a quarter was 5.46 grams so we took the CMU and subtracted it from that and got our answer of 1.16 CMUs.
Using the idea of the CMU we can come up with a conclusion of how scientist figured out the atomic mass unit. They probably took the most common measurement of atoms and set that as the Atomic mass unit then that would be the bases of every measurement.
We decided to use the Nickel as the CMU which meant that a CMU weight would be 4.72 grams. The mass of a quarter was 5.46 grams so we took the CMU and subtracted it from that and got our answer of 1.16 CMUs.
Using the idea of the CMU we can come up with a conclusion of how scientist figured out the atomic mass unit. They probably took the most common measurement of atoms and set that as the Atomic mass unit then that would be the bases of every measurement.